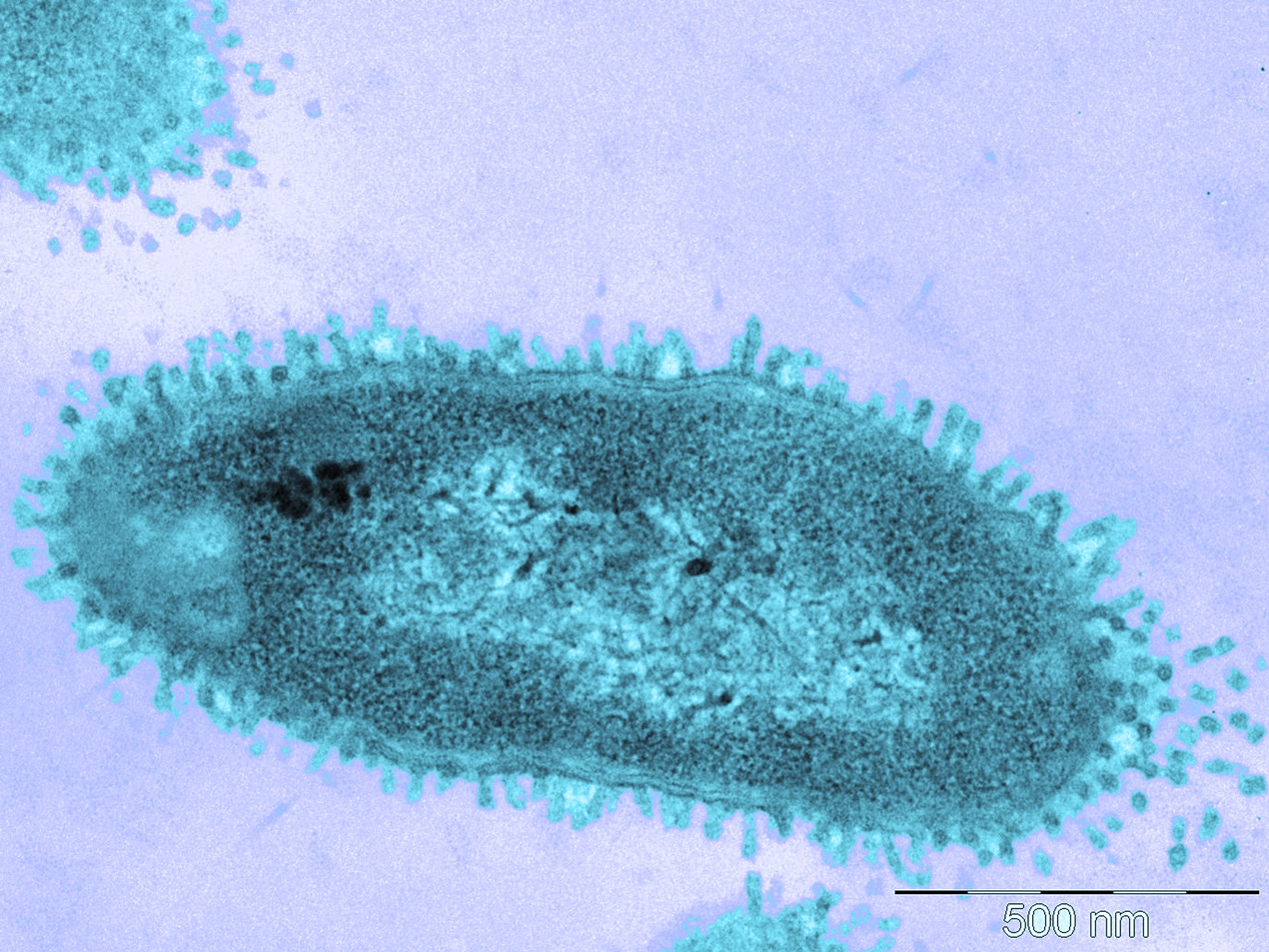
UB researchers develop a compound to create new antibiotics against multi-resistant bacteria
A UB team led by Francesc Rabanal, professor at the Department of Organic Chemistry of the Faculty of Chemistry, has synthesised and developed one of the seven compounds that have reached the final phase of the ENABLE (European Gram Negative Antibacterial Engine) project. This is a programme financed by the IMI (Innovative Medicines Initiative) with the aim of developing new antibiotics for the treatment of infectious diseases caused by resistant and multi-resistant Gram-negative bacteria. A total of forty-seven European universities and companies, led by the pharmaceutical multinational GlaxoSmithKline and the University of Uppsala, are part of this ambitious consortium, which has a budget of EUR 85 million and involves twelve European countries. Since its launch in 2014, ENABLE has evaluated twenty-five molecules synthesised at the best European research centres. The promising drug developed by the UB is based on a natural compound from the polymyxin family, an antibiotic naturally produced by the bacterium Paenibacillus polymyxa.
With two million patients contracting hospital infections each year in the European Union, and 25,000 yearly deaths caused by these, bacterial resistance to antibiotics is an increasingly important health problem. Moreover, in the case of antibiotics, the scientific and economic challenges involved in developing any drug add to the constraints on their use, as healthcare systems restrict new antimicrobial agents reaching the market to those related to the most severe infections in order to prevent bacteria from generating resistance, making it difficult for these antibiotics to be economically viable.
Last resort drugs
Polymyxins are considered as last resort drugs that are used when other antibiotics do not work, as they also present toxicity problems, mainly affecting the kidneys. The UB team’s innovative compound aims to avoid these negative effects by modifying its structure to facilitate its metabolisation and elimination once the compound has performed its antimicrobial activity. “The nephrotoxicity tests on mice that we have carried out as part of ENABLE show that the compound is effective and that it does not accumulate in the kidney”, Dr Rabanal points out.
In addition, according to initial results, the compound has shown efficacy and less toxicity against the Gram-negative bacteria Pseudomonas aeruginosa —which causes infections in hospitals because of its ability to thrive even on surfaces such as medical catheters— and Klebsiella pneumoniae, which causes about 1% of multi-resistant bacterial pneumonias. These bacteria have
been respectively classified as ‘serious’ and ‘urgent’ threats by both the US and European Centers for Disease Control and Prevention.
Proof-of-concept study in an experimental mouse model
Researchers are currently conducting an in vivo proof of concept. That is, they are analysing the efficacy and toxicity of the potential new drug, the optimal dose, the most appropriate route of administration and the type of infection against which it may be useful in an experimental mouse model.
This is the Hit to lead (H2L) stage —or pre-candidate prototype process— one of the initial phases for the creation of a new drug, which is a long and costly process in which only one in 10,000 molecules reaches clinical consideration. It takes between ten and fifteen years of research and development from the moment a candidate is identified and synthesised to the completion of all efficacy and safety testing in the laboratory, in animal models and in humans.
The researchers’ objective is to move on to the Lead to candidate stage, which precedes the first clinical trials, before the end of the project in July 2021. At this stage, the analyses of toxicity, doses and routes of administration are carried out using a more complex experimental animal model, such as a pig model. This qualitative leap implies much higher production costs, as the entire candidate synthesis must be scaled up and infrastructure requirements also increase.
Among the other compounds under development within the ENABLE framework, one has already proceeded to Clinical Phase I (first trials in humans, generally in small groups of no more than one hundred healthy individuals), two have reached the Lead to candidate phase, and three others have reached the Hit to lead phase together with the UB compound.
A model of collaboration of excellence
The ENABLE consortium is an innovative collaborative model for antibiotic discovery. It brings together the skills and experience of the public and private sectors to create a powerful platform of excellence for antibacterial drug discovery.
“The project works as a drug development engine that puts at your disposal all the necessary infrastructure to advance the process: every three months a committee of international experts evaluates the progress of candidate compounds which, depending on the results, are either discarded or sent to the different platforms indicated for each phase of development. These platforms are located in the various countries forming the European consortium,” explains Francesc Rabanal. “Participating in this consortium,” continues this researcher, “has been very important, because developing a new antibiotic is a very time-consuming and resource-intensive task, and European coordination accelerates the development process. ENABLE is part of the New Drugs for Bad Bugs (ND4BB) programme, a series of projects aimed at overcoming existing obstacles to the development of new antibiotics. This programme is promoted by the IMI, the largest public-private initiative in Europe, with a budget of EUR 2 billion, formed by the European Commission and the main pharmaceutical companies (through EFPIA, the European Federation of Pharmaceutical Industries and Associations).
The FBG has managed the patent application protecting Dr Rabanal’s family of compounds, which has been selected to participate in this project; it has carried out the negotiation with the other partners of the consortium regarding the industrial property rights of the project’s results, and it is looking for potential licensees for this technology. In addition, the FBG-UB international research projects office has undertaken the financial management of the project.

