Nanotechnological eyedrops for glaucoma, dry eye and ocular inflammation
Product technical advantages
- Biocompatible and biodegradable nanoparticles
- Novel ocular delivery system with improved stability
- Sustained release and enhanced bioavailability
- Easy industrial scale-up
- Patient-friendly
Product benefits
- Neuroprotection
- Reversion of prostaglandins side-effects (dry eye reversion and anti-inflammatory effect)
- Long-term effects: less eyedrops to administer with enhanced effects
- Other potential therapeutic effects: antibacterial, anti-microbial effects
- Possibility to incorporate a prostaglandin or other drugs to replace current glaucoma medications
Goal
The group is looking for a license agreement.
Intellectual property
Product patented (PCT/EP2024/052923)
Reference
UBTT0430
Contact
Rosa Vázquez
Email: rvazquez@fbg.ub.edu
Tel: +34 661749010
Nanotechnological eyedrops for glaucoma, dry eye and ocular inflammation
Problem addressed by the project
Nanotechnological eyedrops are focused on glaucoma and associated dry eye and ocular inflammation. Glaucoma total annual costs to NHS in Spain account for €10 million. Our product will significantly decrease this burden.
In Spain, 728,893 patients diagnosed with glaucoma and 25% of them not-adhere to treatment (182,224 patients).
Moreover, 50% of the patients experience side effects with current treatments, which are dry eye and ocular inflammation. Also, no current medication addresses neuroprotection.
Addressing neuroprotection and overcoming adverse effects may increase adherence rates, extend surgery timespan and increase quality of life for glaucoma patients.
Glaucoma market
It is estimated that by 2040, 22 million people will suffer vision loss due to glaucoma.
Medication discontinuation rates are alarmingly high (25%), mainly due to non adherence.
Current medications cause adverse effects, do not exert neuroprotection and 15% of patients end up having surgery after 5 years of diagnosis.
There is an unmet medical need for:
- Development of neuroprotectant agents to overcome glaucoma progression.
- Overcome dry eye and ocular inflammation adverse effects of current medications increasing drug adherence.
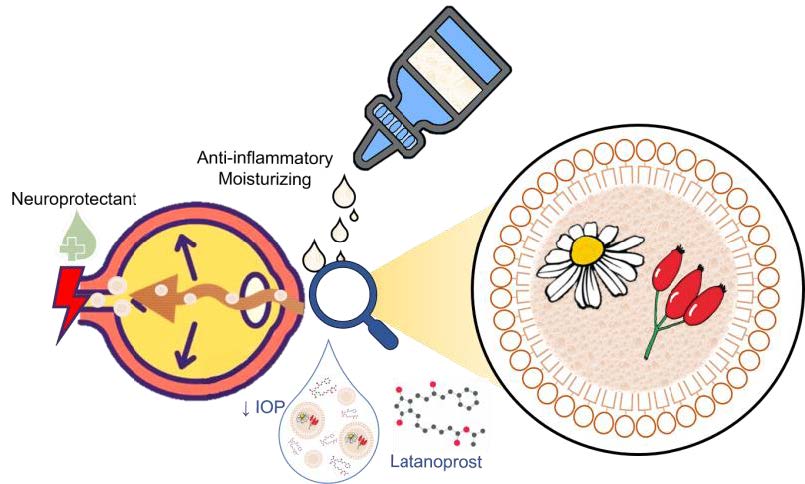
Product
Ophthalmic eyedrops classified as medical device formed by second-generation nanostructured lipid carriers (NLCs) containing natural compounds offering:
- Retinal and optic nerve neuroprotection effective for glaucoma
- Reversion of marketed medication side-effects
– Therapeutically effective against dry eye
– Anti-inflammatory
Project publications
Int J Pharm . 2024 May 11:658:124222.
doi: 10.1016/j.ijpharm.2024.124222
Int J Nanomedicine . 2023 Nov
23:18:6979 6997. doi: 10.2147/IJN.S429565

