SRC INHIBITORS AND USES THEREOF
Benefits
This technology provides several benefits such as:- Inhibiting Src specifically in cancer cells addresses the issue of treatment-associated toxicity and the limited dosage allowed for current Src inhibitors.
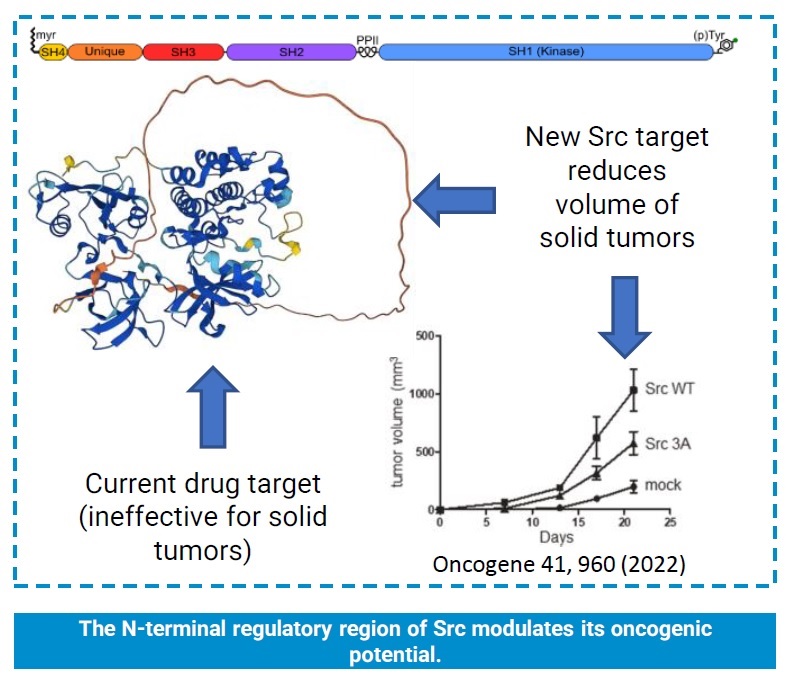
- Mutations in the N-terminal region of c-Src have led to a 50% reduction in Src-dependent tumor growth.
- Utilizing click chemistry for synthesizing new molecules provides advantages in efficiency, selectivity, versatility, speed, and compatibility.
Objective of the collaboration
The represented institutions are looking for a potential partner to co-develop the technology through a licensing agreement of the patented procedure. An exploratory agreement to further explore the efficacy, safety, clinical relevance, and market potential of drugs targeting the intrinsically disordered region of Src for cancer treatment would be an acceptable first step. However, the form, terms, and conditions of the collaboration can be openly discussed if the presented technology is of interest.Intellectual Property
A European Patent Application has been submitted in 2024 (EP24382104.8).Reference
UBTT0483Contact
Isabel Durán Email: iduran@fbg.ub.eduSRC INHIBITORS AND USES THEREOF
The Challenge
According to the World Health Organization, cancer is a leading global cause of death, affecting 20% of men and 17% of women. Current treatment modalities include surgery, radiotherapy, and systemic therapy, chosen based on cancer type and extensive research. Src, a tyrosine kinase protein, plays a pivotal role in dysregulating cellular pathways linked to tumor development. Despite not being the primary cancer driver, Src influences crucial aspects of cancer cell survival, including metastasis, migration, invasion, stemness, and resistance to chemo and immunotherapy, making it an appealing target for improving survival rates. Although four FDA-approved Src inhibitors primarily target hematological malignancies, they demonstrate limited efficacy against solid tumors like colorectal and breast cancer, where Src overexpression predicts poor prognosis.
Researchers from the BioNMR group, belonging to Universitat de Barcelona (UB) and Universitat Internacional de Catalunya (UIC), have demonstrated that the poorly studied Src Unique Domain (UD) is a regulatory region and a new drug target potentially overcoming the limitations of current Src inhibitors, all of them directed to the Src kinase domain. This novel approach is expected to enhance the efficiency and minimize off-target effects that have prevented so far the development of drugs targeting one of the most relevant oncogenes.
Technology description
The technology focuses on the N-terminal intrinsically disordered region (DR) of Src, an area that has been less studied but holds significant potential as an alternative target to the conventional kinase domain.
Through a combination of biophysical methods, site-directed mutagenesis, cell biology, and in vivo studies, they have uncovered that the DRs of Src act as regulatory elements, facilitating the formation of liquid-liquid separated phases or protein condensates. This novel finding opens up new avenues for therapeutic intervention, as targeting these condensates could offer a more effective approach compared to traditional Src inhibitors.
One of the primary reasons for the limited success of current Src inhibitors lies in their off-target effects, often inhibiting other kinases with similar binding pockets, leading to toxic side effects and dosage limitations. By focusing on the unique sequence of the DR, their approach aims to minimize off-target effects and enhance selectivity towards cancer cells overexpressing Src.
Additionally, the dependence of condensate formation on protein expression levels suggests that drugs targeting these condensates would predominantly affect cancer cells, offering the potential for reduced toxicity and improved efficacy in cancer treatment.
Current stage of development
- New drugs reduce growth, proliferation and migration in cells.
Future Milestones:
- In vivo studies.
- Cell line dependency.
- Biomarker for early detection of susceptible cell types, lipidomics, etc.
- New drugs within the scope of the patent.

