Novel adoptive CAR-NK cell transfer therapy in refractory invasive fungal infection
Advantages
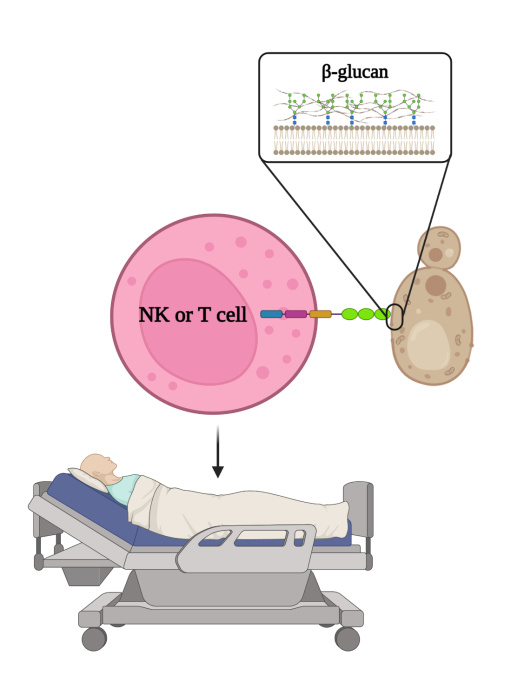
No CAR-based cell therapies directed to fungal infections are clinically available. Our universal CAR-NK approach allows rapid treatment of immunosuppressed patients with invasive fungal infections refractory to antifungals.
Goal
The group is looking for a license agreement, but other collaborations may be considered.
Intellectual Property
Patent pending.
Patent application nr. PCT/EP2022/068416
Reference
UBTT0431-E
Contact
IDIBAPS:
Knowledge and Technology Transfer Office: innova@recerca.clinic.cat
University of Barcelona:
Inma Íñiguez: iiniguez@fbg.ub.edu
Novel adoptive CAR-NK cell transfer therapy in refractory invasive fungal infection
Description
Invasive fungal infections are a cause of high morbidity in immunocompromised patients (e.g., bone marrow transplant recipient, and cancer or ICU admitted patients). Moreover, there is an emergence of fungal infections multi-resistant to available antifungals, which are in turn costly and have significant associated toxicity. All this meaning that mortality in these cases can exceed 70%.
The research group proposes a solution based on Off-the-self allogeneic CD5CAR NK cells for rapid adoptive cell transfer therapy overcoming antifungal drug resistance.
Current stage of development
The CD5 CAR project is pending of AEMPS’s (Spanish Agency for Medicinal and Sanitary Products) approval for initiation of clinical phase I trial by the end of 2023.

